Hairdryer vs. Bowl of Water
Back in 2006, I conducted five experiments which tested thermal properties and behaviors of ice and water.
They were:
1. What will melt faster, ice in a bowl or ice in a colander?
2. Which will freeze faster, boiling hot water or cold water?
3. Which appliance will melt ice faster, A microwave oven or a stove?
4. Which will melt ice faster, an incandescent bulb or a candle?
5. Which will melt faster, ice in a cardboard box or in a refrigerator?
I was excited to conduct and record the results of these experiments because I really didn't know the answers before I started.
So often in books and on the web, I read about experiments or studies that were conducted to find out if hypotheses were correct. That's actually closer to how science is done. These ice melt experiments were done purely as a learning exercise. I didn't have anything at stake... except perhaps a slight inclination towards unexpected results.
Last weekend, six years after the original experiments, I tried one more.

The first one of the previous experiments impressed upon me how effective liquid water is at conducting heat. It literally changed how I behave in real life, particularly in the kitchen.
Want to cool off? Jump in cold water. Want to thaw a frozen turkey? Submerge it in water.
I was also inspired to conduct another experiment. The experiment below.

Which would melt an ice cube faster? A 1600 Watt hairdryer or a bowl of water?
Here's the setup. The bowl was a two-quart bowl of water. This seemed like enough water to absorb an ice-cube worth of coldness without significantly lowering the water temperature. The hairdryer is electric. I'll run it on HIGH temp, full power, directed towards a single ice cube. The ice will be on a steel grating, so melted ice flows away and the dryer air won't push the ice around.

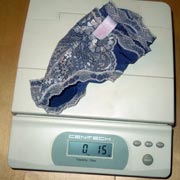
Both ice cubes were identical, to 1/10th of a gram.

At precisely the same moment, we started the melt, pointing a hairdryer at Ice Cube A, dropping Ice Cube B into the bowl of water.

Side by side, they endured the heat. The ice under the hair dryer was being hit by a much hotter melting force, but the ice in the water bowl was immersed in its enemy, melting away on all sides.

Who would triumph in this endurance test to stay solid?

At the 4½ minute mark, the ice cube in the bowl of water was gone. Completely melted, it was reduced to a ring of bubbles on the surface of the water.
We shut off the hair dryer and assessed the remaining ice.

The hairdryer had done well, but it simply couldn't melt the ice as fast as a nice room-temperature bath. It had lost to the water bowl.
A one gram chip of solid ice remained, unmelted by the dryer.
Video
The video below illustrates the test above, but it is not a very watchable video, due to the eccentric camera work and hairdryer drone. Watch at your own risk.
Conclusion
Submerging frozen things in tap water is a terrific way to defrost or melt them. It's a great technique for frozen chicken or broccoli, but not appropriate for corn dogs.
Cockeyed home page | Contact | Terms and Conditions | Updated August 18, 2012 Copyright 2012 Cockeyed.com