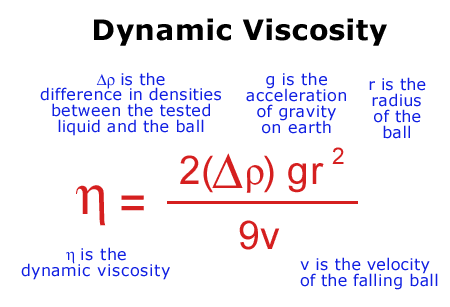
It is a modified configuration of Stoke's Law.
I found it on a University of Hawaii page, a University of Madison, Wisconson page and in other places.
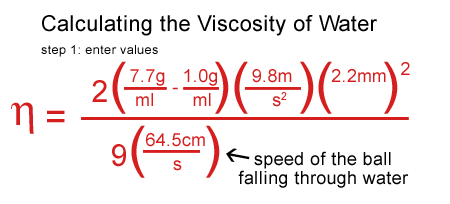
I plugged my values for water in, including the density of the water, the density and the speed of the ball.
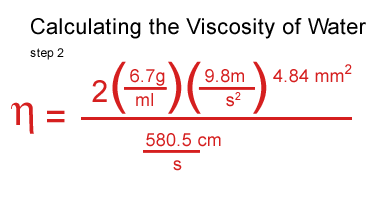
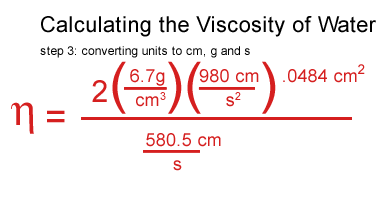
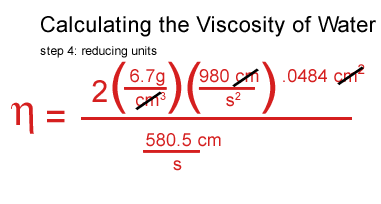
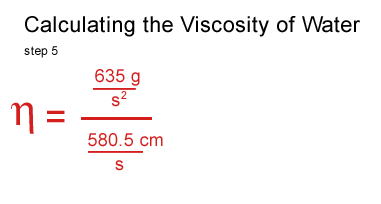
I got stuck at this point and consulted a real scientist & cockeyed.com supporter, John O'Meara at the Center for Astrophysics & Space Sciences at UCSD.
He was operating without the benefit of Goldschlager, but coaxed me forward in my calculations.
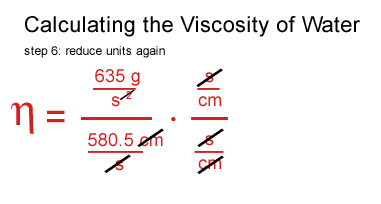

John arrived at the same numbers and was as perplexed as I am.
I seem to be off by a factor of 100.
I used this technique to calculate the incorrect viscosity for the eleven other liquids too.

